Case Report | Vol 11 | Issue 2 | May-August 2025 | Page: 17-22 | Deepika Rani Basappakokati, Shashikant Shankar Swami
DOI: https://doi.org/10.13107/jaccr.2025.v11.i02.278
Open Access License: CC BY-NC 4.0
Copyright Statement: Copyright © 2025; The Author(s).
Submitted: 19/07/2025; Reviewed: 25/07/2025; Accepted: 30/07/2025; Published: 10/08/2025
Author: Deepika Rani Basappakokati [1], Shashikant Shankar Swami [2]
[1] Department of Anaesthesia, Mater Misericordiae University Hospital, Dublin, Ireland.
[2] Pain Medicine Unit, St. Vincent’s University Hospital, Dublin, Ireland.
Address of Correspondence
Dr. Deepika Rani Basappakokati ,
Department of Anaesthesia, Mater Misericordiae University Hospital, Dublin, Ireland.
E-mail: dr.deepika.rani.b@gmail.com
Abstract
Background: Congenital tracheal stenosis (CTS) is an uncommon condition that is typically diagnosed in infancy. Cases in adults are infrequent and present diagnostic and treatment challenges, especially if there have been previous airway interventions or accompanying anomalies.
Case Presentation: We report the case of a woman in her early twenties with a history of CTS, previously treated in childhood with dilatations and laryngotracheal reconstruction. She presented in adulthood with progressive dyspnoea, exertional fatigue, and presyncope. Imaging and bronchoscopy identified a 3 cm distal tracheal stenosis (7–8 mm in diameter) just above the carina, accompanied by bronchus suis and double superior vena cava. The patient underwent scheduled slide tracheoplasty with cardiopulmonary bypass intraoperatively. Had an uneventful postoperative period.
Discussion: This case report highlights the challenges involved in diagnosing and managing CTS in adults. We give a short overview of existing research on its development, symptoms, how to diagnose it, and treatment choices, focusing on how slide tracheoplasty helps with long-segment stenosis.
Conclusion: CTS may occasionally manifest in adulthood and should be considered in patients with chronic respiratory symptoms and prior airway surgeries. Slide tracheoplasty remains the surgical treatment for long-segment CTS and can produce excellent outcomes even when performed later in life.
Keywords: Congenital tracheal stenosis, slide tracheoplasty, long-segment stenosis, bronchus suis, Tracheal stenosis
Introduction
Congenital Tracheal Stenosis (CTS) is a rare but serious condition that typically occurs in newborns or young infants, presenting symptoms like stridor or respiratory distress. It occurs in approximately 1 in 64,500 [23] live births, with a mortality rate ranging from 44% to 79% [1]. The prognosis mainly depends on the severity of the stenosis and associated anomalies.
This review provides a comprehensive analysis of congenital tracheal stenosis, emphasising its management in adult patients, supported by a unique case from our clinical experience.
Case History
A woman in her early twenties with a history of congenital tracheal stenosis (CTS), diagnosed during infancy following repeated respiratory infections. In the sixth month, a bronchoscopy showed grade 2 subglottic stenosis (Cotton-Myer classification), requiring multiple dilatations and a laryngotracheal reconstruction with rib cartilage at age one.
Throughout her childhood and teenage years, she experienced recurrent lower respiratory infections and stridor, which required the use of antibiotics and, occasionally, steroids. Alongside conservative management, she regularly underwent tracheal dilatations—initially once or twice a year, increasing to every three months during late adolescence. In October 2024, due to worsening subglottic stenosis, she had a tracheal resection with end-to-end anastomosis.
Following this, she developed worsening symptoms, including shortness of breath, fatigue, fainting, and exertional difficulty. A chest CT scan revealed a 3 cm long narrowing of the distal trachea, measuring 7–8 mm in diameter. This narrowing was located just above the carina and beyond a bronchus suis originating from the right upper lobe. Additionally, a double superior vena cava was detected.
The patient was referred to our centre for further assessment and management. A rigid bronchoscopy confirmed a stenosis in the distal airway with a normal proximal trachea. She successfully underwent slide tracheoplasty under cardiopulmonary bypass.
Postoperatively, she was transferred to the Intensive Care Unit(ICU) for continued care and extubated on the same day. After an uneventful four-day stay in the ICU, she was discharged to the High Dependency Unit (HDU). Eventually, she was discharged home on the tenth postoperative day. During the virtual follow-up (two weeks after discharge), she reported notable improvements in respiratory function and exercise capacity.
Tracheal stenosis
Tracheal stenosis refers to an abnormal narrowing of the tracheal lumen below its normal size. It may be congenital or developed after an airway injury.
Embryology of the Trachea
The formation of the lower respiratory tract begins on day 22. It has five stages: embryonic, pseudoglandular, canalicular, saccular, and alveolar. Full maturation occurs around the age of 8.
The respiratory system develops from a diverticulum of the foregut endoderm, which splits into the trachea and oesophagus. By the fourth week, the trachea forms bronchial buds, branching into secondary and tertiary bronchi to create lung lobes by week six. From weeks five to seven, the mesoderm develops into the pleura, shaping the thoracic and abdominal cavities.
Lung development occurs in stages: during the pseudoglandular stage (weeks 5–17), the bronchial tree branches, forming glands, cilia, arteries, cartilage, and smooth muscle. However, infants can’t survive without surfactant.
In the canalicular stage (weeks 16–25), respiratory units and capillary networks develop, with type II pneumocytes beginning surfactant production around week 20; gas exchange remains limited.
The saccular stage (from 24 weeks until birth) involves lung expansion into saccules and the maturation of pneumocytes for gas exchange. Surfactant production begins at 24 weeks but isn’t sufficient until about 32 weeks, which affects viability.
During the alveolar stage (from 36 weeks to 8 years), alveoli develop from the secondary septa, growing until full maturity at age eight and completing respiratory development.
Anatomy of the Trachea
The trachea begins at the lower larynx and extends to the bronchi at the carina. It is wider at the top and consists of 16–20 C-shaped cartilage rings connected by a thin membrane. The posterior wall of the trachea is adjacent to the oesophagus and contains the trachealis muscle, which helps facilitate coughing and clearing secretions.
In adults, its diameter is 15-20 mm and its length 10-11 cm, which is wider in males
The trachea has four layers: 1. mucosa with ciliated epithelium and goblet cells; 2. submucosa with nerves, vessels, elastin, and collagen; 3. smooth muscle; hyaline cartilage rings for support; and 4. outer adventitia attaching it to surrounding tissues.
Pathophysiology of Congenital Tracheal Stenosis
CTS is an uncommon condition. Among these cases, 10–25% are isolated CTS, while 75–90% are associated with other congenital anomalies. There is a higher prevalence in males and a strong link with prematurity [24, 25].
Congenital tracheal stenosis may be caused by:
1. Complete tracheal rings, with a missing posterior membranous trachea replaced by complete cartilaginous rings. This rare anatomical feature occurs in about 1 in 50,000 cases.
2. Tracheal cartilaginous sleeve, where cartilage forms a sleeve instead of rings, increasing the risk of airway obstruction.
Classification of CTS:
Various tracheal stenosis classification systems reflect causes, presentations, and severity. These are categorised by anatomy, extent of narrowing, mortality risk, congenital or acquired, intrinsic(or extrinsic), and length.
Cantrell and Guild [2] first classified congenital stenosis into generalised hypoplasia, funnel-shaped, and segmental types. Hoffer et al. [3] later refined this by adding segment length and associated anomalies, affecting treatment and outcomes:
Class 1: Short-segment (<30%), good prognosis (~8% mortality).
Class 2: long segment (>50%), no significant anomalies (~45% mortality).
Class 3: Associated with cardiopulmonary malformations (~79% mortality).
Since this lesion is mechanical, medical management is often ineffective (~40% survival rate) and linked to a poor prognosis [4]. Surgical correction is the primary treatment. Significant cardiac anomalies usually influence surgery timing and outcome.
Commonly Associated Anomalies:
• Cardiovascular(Left pulmonary artery sling, PDA, VSD, Double aortic arch, Aberrant subclavian artery
• Respiratory: Pulmonary agenesis or hypoplasia, Tracheal bronchus
• Other Systems: Gastrointestinal malformations, Renal anomalies, Skeletal abnormalities
Clinical Presentation
A high degree of suspicion is crucial in diagnosing CTS in infants and children with respiratory distress. Tracheal stenosis often presents as biphasic stridor within weeks of birth. Inspiratory stridor stems from cervical trachea stenosis, while expiratory stridor stems from thoracic narrowing or collapse.
Symptoms include a brassy, non-productive cough, ‘washer machine’ breathing, nasal flaring, wheezing, intercostal retractions, pursed lips, persistent croup, and intermittent cyanosis. Due to increased breathing and feeding effort, infants may hyperextend their necks or fail to thrive. A 1-mm airway reduction causes a 44% decrease in cross-sectional area.
Symptoms often do not appear until 50% stenosis, with dyspnoea at 75% stenosis [7]. Presentation usually worsens with respiratory infections, leading to reflex apnoea or life-threatening spells. Typical birth signs include no audible cry, difficult intubation, and respiratory distress.
Hiyama et al. [5] described two patients suspected of respiratory distress without an audible cry and difficulties in intubation. Typical symptoms of tracheal stenosis at birth include the absence of cry, difficult intubation, and respiratory distress [6].
Diagnostic Evaluations
1. Air tracheograms and fluoroscopy: Reveal accurate details about tracheal anomalies and their locations.
2. Imaging: Provides detailed information about the cross-sectional area and extent of lesions.
Spiral CT is particularly beneficial because it’s a quick procedure, which is especially important for young patients. MRI offers similar information and is particularly useful for identifying associated cardiovascular anomalies.
3. Virtual endoscopy uses helical CT to create airway reconstructions, hence detecting the stenosed areas. It’s a non-invasive method that benefits patients who are intolerant to bronchoscopy with about 95.5% accuracy for central airway stenosis. However, it is less effective at segmental airways and may miss a lesion size of less than 1.5 mm.
4. Bronchoscopy:
A. Rigid bronchoscopy under general anaesthesia remains the gold standard for diagnosing and assessing the length and diameter of CTS. Still, it can be challenging to visualise the stenotic segments fully.
B. Flexible bronchoscopy serves as an alternative to visualising the narrow tracheal segment. Since mucosal oedema can cause severe airway obstruction, careful insertion is essential, ideally performed by experienced bronchoscopists familiar with CTS.
4. Although angiography is used less often, it remains the gold standard method for accurately identifying vascular anomalies.
5. Echocardiography: useful for identifying cardiovascular issues/anomalies.
6. Barium swallow: an important diagnostic method for detecting a vascular ring or aberrant subclavian artery.
7. Pulmonary function tests: For undiagnosed CTS cases in childhood or adulthood, it’s really important to perform pulmonary function tests. These assessments assist us in identifying problems with airflow and understanding the functioning of the respiratory system.
Newer diagnostic techniques
Recent advances in medical technology have greatly improved diagnostics process. Several newer diagnostic methods are increasingly being used to classify CTS diagnosis and subsequent surgical planning.
1. Endobronchial ultrasound (EBUS): This technique provides real-time imaging of the airways, allowing clear visualisation of lesions and tissues.
2. Photoacoustic endoscopy (PAE) uses laser-generated ultrasound for detailed tissue imaging, potentially identifying malignant lesions, especially in high-risk patients.
3. Optical coherence tomography (OCT) visualises tissue microstructures in real time. Its role in diagnosing tracheal stenosis is limited.
4. Confocal laser endomicroscopy (CLE) provides high-resolution, cellular-level tissue images. However, cost and special training limit its use.
Differential diagnoses
1. Tracheomalacia It involves a floppy trachea that collapses during exhalation, unlike tracheal stenosis, which is fixed narrowing.
2. Subglottic stenosis narrows below the vocal cords, leading to stridor.
3. Vascular rings: This is a congenital anomaly characterised by abnormal vessels that encircle and compress the trachea or oesophagus.
4. Mediastinal Masses can compress the trachea, similar to tracheal stenosis; imaging like CT helps differentiate.
5. Other Congenital Anomalies, such as laryngomalacia, vocal cord paralysis, or oesophageal atresia, can mimic tracheal stenosis symptoms.
6. Acquired Tracheal Stenosis, caused by trauma, inflammation, etc..
Management
Over the past twenty years, surgical and endoscopic advancements have improved patient outcomes. The main goal is to lower high complication rates, not just focus on survival. Not all tracheal stenosis patients need surgery; asymptomatic ones can often be monitored and may not require intervention [10]. A better understanding of the condition helps in tailoring the treatment based on symptom severity, as shown in Figure 1.

Conservative Management
With high-resolution imaging, milder or subclinical tracheal stenosis is detected more often. Conservative treatment is advised for mild symptoms. While some cases need intervention, studies indicate others can be safely monitored without surgery. Due to the risks of tracheoplasty, non-surgical options like anti-reflux medications, antibiotics, physiotherapy, and humidified air are increasingly favoured.
A conservative approach is recommended for selective tracheal stenosis cases, especially mild symptoms or complete rings. A study following six patients over 10.6 years found that stenotic tracheas can naturally catch up, with airway diameter normalising around age 9 [11].
Rutter et al. [12] studied 10 patients with complete tracheal rings for at least 12 months. Five showed tracheal growth and symptom resolution, two needed tracheoplasty, and three were too young to determine outcomes. These findings suggest that up to 10% may not require surgery. Observation is a safe option in mild cases, assisting in decisions regarding surgery. Delaying surgery can also be more manageable in older infants [12].
However, this approach has its own risks. Common viral upper respiratory infections can worsen airway obstruction, potentially endangering patient safety. In a systematic review, Ywakim and El-Hakim [14] reported a 5% mortality rate among studies evaluating conservative treatment.
Endoscopic Treatment
Endoscopic treatments are minimally invasive procedures used to treat tracheal obstructions. These methods include laser excision, dilation, and stent placement, and they are often combined with topical steroids or mitomycin C to help ensure the best results. These techniques effectively address tracheal webs and other airway abnormalities.
a. Laser: The CO₂ laser is preferred due to its limited thermal effect, while Nd:YAG is mainly used for haemostasis.
b. Balloon dilation expands outward, reducing trauma and helping with repositioning. Although it can sometimes eliminate the need for open tracheoplasty, it also carries risks such as tracheitis, pneumomediastinum, tracheal injury, stent displacement, and even death. More research is needed to assess its long-term efficacy.
c. Intraluminal stents are used to treat congenital stenosis before or after surgery. They can become integrated into the trachea, making removal difficult, and may cause granulation tissue growth. Ballooning can address this issue, but the long-term outcomes are still uncertain. Absorbable stents are currently being tested for potential future application.
Open Surgical Management
Advances in surgical techniques have greatly improved how we treat congenital and acquired tracheal stenosis, including severe forms like long-segment stenosis. Surgery is usually recommended for CTS patients who are facing respiratory failure or life-threatening events. Cardiopulmonary bypass and ECMO support help maintain stability during procedures, while related cardiovascular anomalies are often corrected at the same time.
For short-segment CTS, resection with end-to-end anastomosis is preferred, while slide tracheoplasty remains standard for long-segment cases, applicable across all types and usually has low postoperative complications. However, it carries risks, with mortality rates from 5% to 20%, mainly due to restenosis, granulation tissue, or tracheobronchomalacia.
Short-segment CTS
Resection and Anastomosis
For short-segment tracheal stenosis involving less than 30% of the trachea or fewer than 6–8 rings, tracheal resection with primary anastomosis is preferred, with 1–8% mortality [2]. This procedure has high success rates when used for acquired lesions and congenital stenoses. With the mobilisation of both segments, up to 30% of the trachea can be safely excised
Grillo et al. [13] reported on 503 patients with postintubation stenosis, with 87.5% symptom-free, 5.7% dehiscence, and 2.4% mortality. Other single-centre studies show mortality from 0% to 16%, highlighting the importance of patient selection [26, 27]. (Fig. 2)

Long Segment CTS
Long-segment tracheal stenosis presents unique surgical challenges, primarily due to the excessive tension placed on suture lines during repair, which can lead to high complication and failure rates. Grillo, his colleagues, and other groups have demonstrated this in their seminal works over the past decades [15, 16-20] . Techniques like pericardial patch tracheoplasty and autograft or homograft reconstructions have been developed to address these limitations. Slide tracheoplasty is preferred, as it reduces anastomotic tension and provides a more effective and durable repair.
1. Slide Tracheoplasty
Slide tracheoplasty, first performed in 1989 by Victor Tsang and Peter Goldstraw for complete tracheal rings [22], has been refined and is now the preferred method for long-segment CTS. It enlarges the airway with native tracheal tissue, offering stability, reducing granulation risk, and promoting quick healing. It preserves the continuity of the epithelium, which helps in better healing.
It involves dividing the stenotic segment at the midpoint, with vertical incisions It involves dividing the stenotic segment at the midpoint, with vertical incisions along the upper and lower segments posteriorly, allowing them to slide over each other for a wider, more stable result airway. (Fig. 3)

2. Patch Tracheoplasty
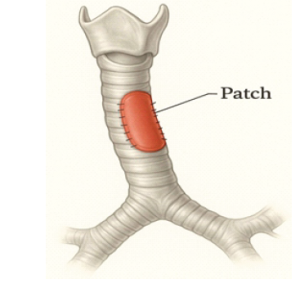
While slide tracheoplasty is standard for long-segment CTS, it isn’t suitable for all cases, especially those involving bronchial abnormalities like a pig bronchus (bronchus suis) or stenosis extending into the bronchial tree. In such cases, patch tracheoplasty is an alternative to reconstructing and enlarging the airway. Materials used include costal cartilage, pericardium, tracheal autografts, and allografts. Synthetic grafts are deserted mainly due to high infection and granulation risks. (Fig. 4)
Complications associated with surgical management
Since complications are common after surgical management, dedicated postoperative care is crucial. Management increasingly relies on endoscopic treatments such as balloon dilation, laser excision, or stent placement, but occasionally a tracheostomy or further surgery remains necessary.
1. Granulation tissue can block airways, especially at graft sites, sutures, stents, or tracheostomies. Treatments include cold excision, laser, balloon dilation, topical steroids and mitomycin C to limit regrowth.
2. Restenosis is common after open tracheoplasty, with risk depending on initial airway narrowing severity (Backer et al. [21]; Grillo et al [16]. Backer et al. reviewed 50 children with complete tracheal rings, reporting reintervention rates of 25% for pericardial patches, 17% for autografts, 0% for resection and anastomosis, and 50% for early slide tracheoplasty. On long-term follow-up, Grillo et al. [16] found no restenosis in eight slide tracheoplasty patients. Management usually involves bronchoscopy, with repeat balloon dilation or stent placement if needed.
Conclusions
Management of congenital tracheal stenosis has improved, with surgery now having low mortality and promising initial results. However, long-term issues often require ongoing airway treatments. Mild cases may be managed conservatively, as some tracheostomies grow naturally. Due to the condition’s rarity, expert teams individualise and guide treatment. Long-term follow-up with bronchoscopy is essential, and new tools like virtual bronchoscopy and tissue engineering are promising.
References
1. Walker LK, Wetzel RC, Haller JA Jr. Extracorporeal membrane oxygenation for perioperative support during congenital tracheal stenosis repair. Aneast Analg. 1992;75:825-829.
2. Cantrell J, Guild H. Congenital stenosis of the trachea. Am J Surg 1964;108:297-305.
3. Hoffer M, Tom L, Wetmore R, et al. Congenital tracheal stenosis. The otolaryngologist’s perspective. Arch Otolaryngol Head Neck Surg 1994;120:449-53.
4. Benjamin B, Pitkin J, Cohen D. Congenital tracheal stenosis. Ann Otol Rhinol Laryngol 1981;90:364-71.
5. Hiyama E, Yokoyama T, Ichikawa T, Matsuura Y. Surgicalmanagement of tracheal agenesis. J Thorac Cardiovasc Surg 1994;108:830-3.
6. Chiu PP, Kim PC. Prognostic factors in the surgical treatment of congenital trachealstenosis:tracheal stenosis: a multicenter analysis of the literature. J Pediatr Surg 2006;41:221–5.discussion 221–225.
7. Lalwani A. Current diagnosis and treatment in otolaryngology.2nd edition. New York: McGraw-Hill Medical; 2007.
8. Koletsis EN, Kalogeropoulou C, Prodromaki E, et al.Tumoral and non-tumoral trachea stenoses: evaluation with three-dimensional CT and virtual bronchoscopy. JCardiothorac Surg 2007;2:18.
9. Hoppe H, Dinkel HP, Walder B, et al. Grading airway stenosis down to the segmental level using virtual bronchoscopy.Chest 2004;125:704-11.
10. Antón-Pacheco JL, Cano I, García A, et al. Patterns of management of congenital tracheal stenosis. J Pediatr Surg 2003;38:1452-8.
11. Cheng W, Manson D, Forte V, et al. The role of conservative management in congenital tracheal stenosis: an evidence-based long-term follow-up study. J Pediatr Surg 2006;41:1203-7.
12. Rutter M, Willging J, Cotton R. Nonoperative management of complete tracheal rings. Arch Otolaryngol Head Neck Surg 2004;130:450-2.
13. Grillo HC, Donahue DJ, Mathisen DJ, et al. Postintubation tracheal stenosis. Treatment and results. J Thorac Cardiovasc Surg 1995;109:486-93.
14. Ywakim R, El-Hakim H. Congenital tracheal stenosis managed conservatively: systematic review of the literature. J Otolaryngol Head Neck Surg 2012;41:288-302.
15. Backer CL, Mavroudis C, Gerber ME, et al. Tracheal surgery in children: an 18-year review of four techniques. Eur J Cardiothorac Surg 2001;19:777-84.
16. Grillo HC. Surgical approaches to the trachea. Surg Gynecol Obstet 1969;129:347-52.
17. Wright CD, Graham BB, Grillo HC, et al. Pediatric tracheal surgery. Ann Thorac Surg 2002;74:308-13; discussion 314.
18. Cotter CS, Jones DT, Nuss RC, et al. Management of distal tracheal stenosis. Arch Otolaryngol Head Neck Surg 1999;125:325-8.
19. Wright CD, Grillo HC, Wain JC, et al. Anastomotic complications after tracheal resection: prognostic factors and management. J Thorac Cardiovasc Surg 2004;128:731-9.
20. Jonas RA, Spevak PJ, McGill T, et al. Pulmonary artery sling: primary repair by tracheal resection in infancy. J Thorac Cardiovasc Surg 1989;97:548-50.
21. Backer CL, Mavroudis C, Gerber ME, et al. Tracheal surgery in children: an 18-year review of four techniques. Eur J Cardiothorac Surg 2001;19:777-84.
22. Tsang V, Murday A, Gillbe C, et al. Slide tracheoplasty for congenital funnel-shaped tracheal stenosis. Ann Thorac Surg 1989;48:632-5.
23. Benjamin B, Pitkin J, Cohen D. Congenital tracheal stenosis. Ann Otol Rhinol Laryngol 1981;90:364-71.
24. Smith MM, Huang A, Labbé M, et al. Clinical presentation and airway management of tracheal atresia: a systematic review. Int J Pediatr Otorhinolaryngol 2017;101:57–64. 10.1016/j.ijporl.2017.07.028.
25. Sokal R, Tata LJ, Fleming KM. Sex prevalence of major congenital anomalies in the United Kingdom: a national population‐based study and international comparison meta‐analysis. Birth Defects Res A Clin Mol Teratol 2014;100:79–91 https://doi.org/ 10.1002/bdra.23218
26. Cotter CS, Jones DT, Nuss RC, et al. Management of distal tracheal stenosis. Arch Otolaryngol Head Neck Surg 1999;125:325-8.
27. Backer CL, Mavroudis C, Gerber ME, et al. Tracheal surgery in children: an 18-year review of four techniques. Eur J Cardiothorac Surg 2001;19:777-84.
| How to Cite this Article: Basappakokati D, Swami SS | Slide Tracheoplasty for Congenital Tracheal Stenosis Presenting in Adulthood | Journal of Anaesthesia and Critical Care Case Reports | May-August 2025; 11(2): 17-22. |
(Article Full Text HTML) (Download PDF)